Presentation Slides from Gilchrist Technical Services Ltd. Alastair Gilchrist-Speaker
Gilchrist Technical Services Ltd.
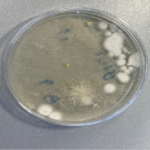
Test result from a batch of cosmetic
product contaminated with mould.
Photograph used by kind permission of Southern Microbiological Services Limited
The 10 Golden Rules Of GM
- Get the facility and equipment design right from the start.
- Practice good hygiene.
- Write good procedures and follow them.
- Identify who does what.
- Train and develop staff.
- Validate processes.
- Keep good records.
- Maintain facilities and equipment.
- Build quality into the whole product lifecycle.
- Perform regular audits an implement improvements identified.
Facility Design
- Design a good logical material flow through the facility.
- Specify the finishes in each area:
- Appropriate to the activity being carried out.
- Which are easy to keep clean and to maintain.
- Ensure that there are changing, hand washing andmessing facilities outside the production areas.
- Provide appropriate storage conditions for the materials,components sub- assemblies and finished products.
- Where possible locate utilities outside production areas.
- Implement and monitor appropriate pest control programmes.
Equipment Design
- Specify and use hygienic equipment.
- Suitable materials of construction.
- Appropriate surface finishes.
- Fully drainable.
- Crevice free.
- Capable of being cleaned and sanitised.
- Write a microbiological control strategy and ensure thatyour equipment design and configuration fits with yourstrategy.
- Minimise the number of operational steps. Make – Fill – Pack
Personnel
- People are your biggest asset.
- Choose them carefully and reward them appropriately.
- Define their roles and make sure that they understand:
- What they have to do.
- How to do their tasks.
- How to record their work.
- Why they have to follow procedures and training.
- Ensure that personnel have the right tools and resources to do thejob.
- Train and develop personnel.
- Tasks can always be done better and improvements should beimplemented.
Microbiological Control Strategy
The war against microorganisms is one which will never be won. Each batch of product is a battle which must be won to ensure the safety andperformance of the product.
- Define how equipment will be cleaned.
- Define how equipment will be sanitised.
- Ensure that the interfaces between equipment is notforgotten e.g. the hose that links the mixer or storagecontainer to the filling machine.
- Document your requirements.
- Write procedures.
- Validate that the procedures meet your requirements.
Raw Materials – the building blocks of cosmetics
- Ensure that appropriate raw materials are used.
- Agree specifications with suppliers.
- Make sure that the specification includes a microbiology limit.
- If the material is known to be hostile to micro organisms e.g. ethanol orsodium hydroxide the specification can be “Not required”
- If raw materials are particularly susceptible tomicrobiological contamination consider basingbatch sizes on full packs.
- Only handle bio-critical raw materials in clean productionareas by trained staff using appropriate sanitised equipment.
- Ensure that part containers of raw materials are re-sealedand stored in an appropriate location.
- Test materials which are susceptible to microbiologicalcontamination.
Water –the most important raw material
- Most cosmetic products which require preservation contain water.
- Water is also the most commonly used medium for cleaning.
- Hot water can be used to sanitise equipment.
- If chemical sanitisation is used water will be required toremove traces of sanitising chemicals.
- City water is not normally considered suitable for makingcosmetic because:
- The quality can be variable [depending on source and/or season] it is not underyour control.
- The water is not purified to remove salts or organic materials.
- Additives such as chlorine or fluoride are often added.
- Purified water to a defined specification should be used toensure consistent product quality and that unacceptable levels of microorganisms are not introduced to the product.
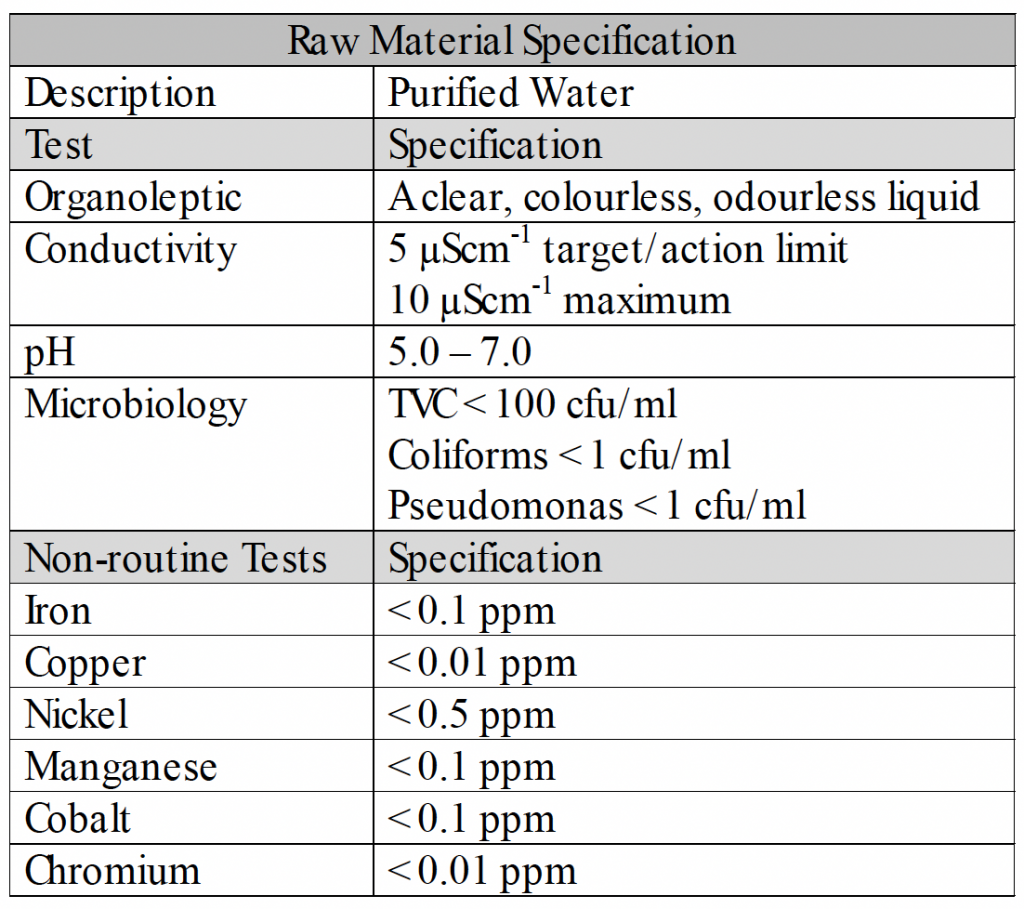
Purified Water Specification
Define the water quality required.
Option 1
• Install a system to produce and store water of the required quality.
• Test the water periodically to ensure that it meets the required quality.
Option 2
• Purchase purified water.
• Use full containers on day of opening or dispose of remains.
Good Hygiene
Clean – no traces of any previous products or cleaning products apart frompurified water.
Cleaning
- Manual – difficult to validate as it is operator dependent.
- CIP – cleaning in place good option for larger items. Easy tovalidate.
- Off-line – dismantle items and clean in a parts washer.Easy to validate. Sanitisation – reduction of viable microorganismsto undetectable levels.
- Hot purified water [> 80°C for > 10 minutes]. No residues. Norinsing.
- Chemical sanitisation. All traces of sanitisers need to beremoved before production starts – creates effluent.Sanitisers need to be rotated.
Sanitisation needs to take place just before production especially if equipment is notdried.
Testing and Validation
Preservative Efficacy Test [PET] or challenge test – every cosmetic product must be assessed to ensure that the preservative system is robust unless itis hostile to micro organisms.
Total Viable Count [TVC] testing should be carried out on:
- Every batch of bio-critical cosmetic product produced.
- Bio-critical raw materials which could contribute to thebioburden of the product [including purified water].
- Correct validated test methodsshould be used. Validation of Processes
- Processes can be validated to reduce testing frequency.
- Testing of validated processes must be continued on an auditbasis.
Photograph used by kind permission of Southern Microbiological Services Limited
Introduction to Cosmetics Preservation with Green Chemistry 12
Comments are closed.